The four basic laws of thermodynamics reveal empirical facts and define physical quantities such as temperature, heat, thermodynamic operations, and entropy that characterize thermodynamic processes and thermodynamic systems in thermodynamic equilibrium.
Table of content
- Zeroth laws of thermodynamic
- First law of thermodynamic
- Second law of thermodynamic
- Third law of thermodynamic
They explain the relationship between these quantities and form the basis for excluding the possibility of certain phenomena, such as constant motion. In addition to its use in thermodynamics, the law is applied interdisciplinary in physics and chemistry.
Zeroth law of thermodynamics

First law of thermodynamic

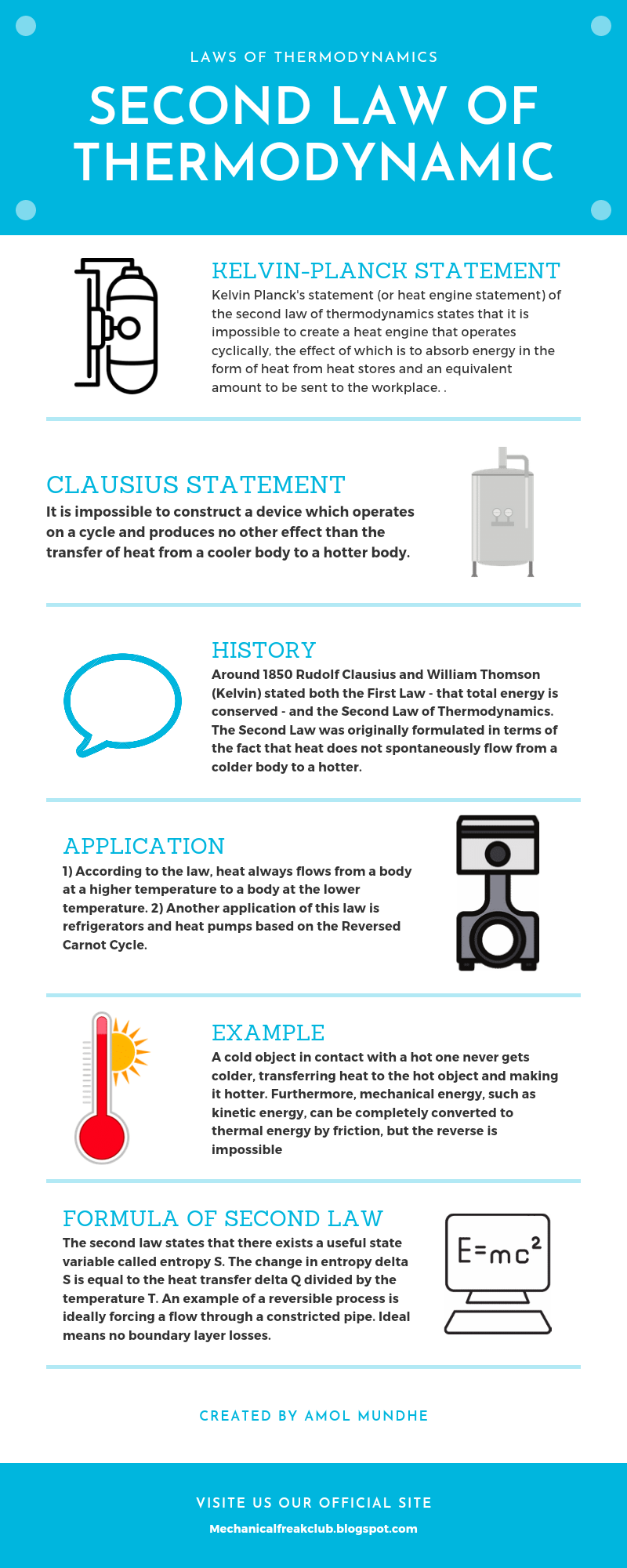
Second law of thermodynamic
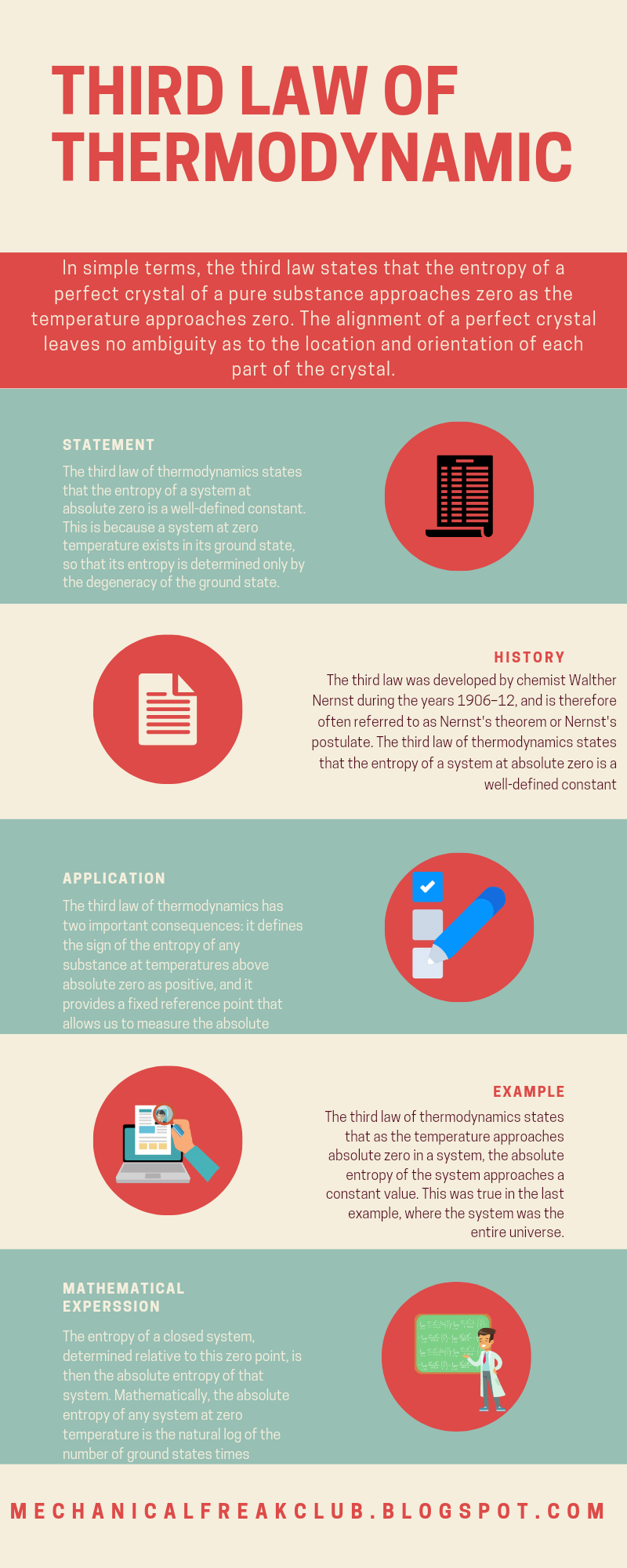
Third law of thermodynamic
Laws of thermodynamics
The four basic laws of thermodynamics reveal empirical facts and define physical quantities such as temperature, heat, thermodynamic operations, and entropy that characterize thermodynamic processes and thermodynamic systems in thermodynamic equilibrium. They explain the relationship between these quantities and form the basis for excluding the possibility of certain phenomena, such as constant motion.